Introduction:
GB261 is a novel and highly differentiated CD20/CD3 bispecific T cell engager antibody computationally designed to maintain Fc effector function, i.e., antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC) to broaden the mechanisms of action (MOA) for tumor cell killing. Furthermore, the “imbalanced” design of GB261 integrates de-tuned CD3 binding to reduce CRS incidence and improve safety features of the Fc effector function. Extensive pre-clinical studies have shown that GB261 has a highly advantageous safety/efficacy balance.Here, we present the preliminary clinical safety and efficacy results of an ongoing phase I/II study for GB261 (NCT04923048).
Methods:
This is an open-label, multicenter (China and Australia), dose-escalation/expansion phase 1/2 study of GB261 to evaluate the safety, tolerability and efficacy in patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma(B-NHL) and Chronic Lymphocytic Leukemia (CLL). Adult patients were eligible when they have CD20+ r/r B-NHL or CLL with no available standard of care treatments, adequate organ function, and no CNS involvement or other CNS disease, infections or other active/serious medical issues that may affect compliance or interpretation of results. GB261 was administered in 21-day cycles, weekly (QW) for the first 6 doses followed by every 3 weeks (Q3W) until disease progression, or other situation defined in protocol. Response assessment was based on Lugano 2014 and LYRIC 2016 criteria.
Results:
As of June 17, 2023, 47 r/r B-NHL patients (DLBCL:76.6%; FL:23.4%) were enrolled at flat or step-up doses of GB261 ranging from 1mg to 300 mg. Median age was 60.0 years (range: 28, 81), 55.3% of patients were male. Median prior lines of therapy were 3 (range: 1, 10). 78.7% of patients were refractory to any anti-CD20 therapy, 70.2% refractory to their last systemic therapy. Median time since last prior therapy to first study treatment was 1.9 months.
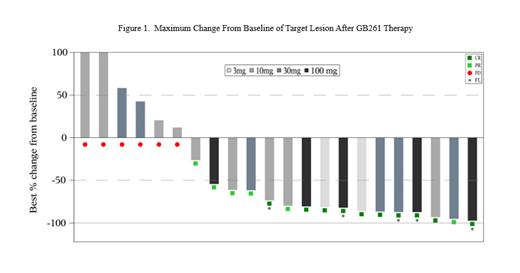
In efficacy evaluable patients (n=22) from 3mg to 100mg, with at least 75% dose exposure before the first radiographic assessment, the median duration of study follow-up was 4.5 months (95%CI: 4.0, 7.4). The overall response rate (ORR) was 73% (16/22), and complete response rate (CRR) was 45.5% (10/22). ORR and CRR were 100% and 100% in 3mg, 56% and 22% in 10mg, 67% and 33% in 30mg. At 100mg dose, there were 5 evaluable patients, with ORR 100% (5/5), CRR 80% (4/5) and PR (20%, 1/5; mosunetuzumab-refractory rrDLBCL patient). Median time to response (TTR) was 1.3 months (95%CI: 1.2, 1.5), the same as median time to CR. Median duration of response (DOR) was not reached.
In safety evaluable patients (n=47), the median duration of study follow-up was 4.1 months (95%CI: 2.9, 5.3). Treatment-emergent adverse events (TEAEs) were 95.7%, treatment-related adverse events were 85.1%. The most common TEAEs were COVID-19 infection (40.4%; grade 1 or 2: 27.6%; grade >=3: 12.8%) and neutropenia (31.9%; grade 1or 2: 14.9%; grade >=3: 17.0%). AE related treatment discontinuation and death were reported in 2 patients, which were all due to COVID-19 pneumonia. CRS occurred in 12.8% (6/47) patients, was mild and transient. CRS in 100mg were 14.3% (2/14). All cases of CRS were grade 1 (8.5%, 4/47) or 2 (4.3%, 2/47), no grade 3 (Lee et al., ASTCT criteria), no interruptions of treatment, and no administration of Tocilizumab. The median duration of CRS was 7 hours. No ICANS were reported.
The PK profile of GB261 appeared to be linear across dose ranges tested (1mg-100mg). Effective half-life appeared to be 2-3 weeks which supports every 3-4 weeks dosing.
Conclusion:
GB261, a novel and highly differentiated CD20/CD3 bispecific antibody, is the first clinical stage Fc+ CD20/CD3 T cell engager. In heavily pretreated B-NHL patients, GB261 showed a highly advantageous safety/efficacy balance, consistent with the MOA. The safety profile is excellent especially for the CRS which is very mild, transient and less frequent compare with other CD20/CD3 bispecific antibodies. The response after GB261 treatment was early, deep and durable. At the 100mg dose level, 80% of patients achieved CR and with favorable safety. Additionally, clinical benefit seen in other CD20/CD3 bispecific antibody failed patients provides clinical support to the unique and differentiated MOA of GB261.
Disclosures
Tan:Janssen: Research Funding; Abbvie: Research Funding; Beigene: Research Funding; Ellipses Pharma: Research Funding; Novartis: Research Funding; Gilead: Research Funding; Genor Biopharma Co. Ltd: Research Funding; Constellation Pharmaceuticals, Inc: Research Funding; Ichnos Sciences SA: Research Funding; Protagonist Therapeutics, Inc: Research Funding; Bristol-Myers Squibb: Research Funding; Qilu Pharmaceuticals Co. Ltd: Research Funding; Loxo Oncology at Lilly: Research Funding; Kartos Therapeutics: Research Funding; Shanghai EpimAb Biotherapeutics: Research Funding. Giri:Royal Adelaide Hospital: Current Employment. Guo:Genor Biopharma Co. Ltd: Current Employment. He:Genor Biopharma Co. Ltd: Current Employment. Xu:Genor Biopharma Co. Ltd: Current Employment. Li:Genor Biopharma Co. Ltd: Current Employment. Zhu:Genor Biopharma Co. Ltd: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal